What is cGDP?
Current Good Documentation Practice (cGDP) refers to a standardized approach for managing documentation in regulated industries such as pharmaceuticals and medical devices. It ensures that records are accurate, reliable, and complete, forming a critical foundation for regulatory compliance and quality assurance.
cGDP is essential to quality management systems (QMS), emphasizing proper documentation throughout a product’s lifecycle—from design to distribution. By following cGDP guidelines, companies can ensure traceability, minimize errors, and prepare for audits and inspections.
Purpose of cGDP
The primary purpose of cGDP is to maintain the integrity of documentation processes, ensure compliance with regulatory requirements, and protect product quality. Proper documentation practices facilitate traceability, reduce errors, and enable proactive risk management. Ultimately, cGDP fosters trust with regulators, customers, and business partners by demonstrating a commitment to high-quality processes.
Regulatory Requirements
FDA Guidelines
The FDA enforces cGDP as part of its Current Good Manufacturing Practices (cGMP) under 21 CFR Part 211. These regulations require that documentation be accurate, complete, and up to date. The FDA emphasizes data integrity and mandates strict controls over records to prevent falsification or errors. Non-compliance can result in warning letters, recalls, or more severe regulatory actions.
EU Guidelines
The European Union also mandates strict adherence to documentation practices in its regulatory framework for medical devices and pharmaceuticals. Agencies like the European Medicines Agency (EMA) require accurate batch records, distribution logs, and testing data to ensure product safety and efficacy. Non-compliance can lead to penalties, market access restrictions, or revocation of marketing authorization.
Ensure Your Documentation Meets Regulatory Standards—Schedule a Consultation with Our Experts Today!
Importance of Data Integrity
Data integrity is fundamental to current Good Documentation Practices (cGDP). It ensures that all documentation, including electronic records, is accurate, complete, and secure from tampering. Proper management of electronic records is essential for tracking processes, replicating activities, and providing reliable documentation during audits.
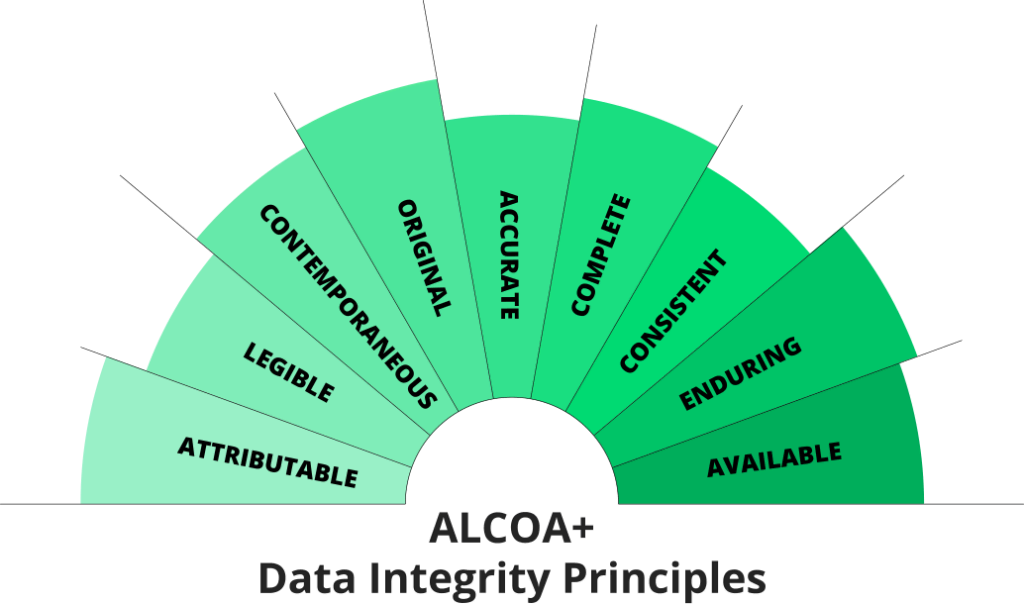
ALCOA+ Standards
The ALCOA+ framework outlines the core principles of cGDP to ensure data integrity. These principles are:
- Attributable: Every entry must identify the individual responsible.
- Legible: Records must be clear, readable, and understandable.
- Contemporaneous: Data should be documented in real time as events occur.
- Original: Records must be authentic and reflect original observations or actions.
- Accurate: All information must be valid and free of errors.
Additional ALCOA+ Elements:
- Complete: Documentation must include all relevant data without omissions.
- Consistent: Processes and documentation methods must follow established procedures.
- Enduring: Records must be preserved securely for the required retention period.
- Available: Documentation should be accessible for audits, reviews, or operational needs.
Benefits of Implementing cGDP
- Improved Quality Assurance: Ensures consistent and reliable processes, reducing errors and enhancing product quality.
- Regulatory Compliance: Facilitates adherence to FDA, EMA, and ISO standards, minimizing the risk of penalties or recalls.
- Increased Efficiency: Streamlines operations by reducing rework and ensuring uniformity in documentation processes.
- Enhanced Patient Safety: Accurate records help trace issues quickly, reducing risks and improving outcomes.
- Reduced Legal Liability: Comprehensive records protect against legal repercussions by demonstrating compliance and accountability.
Documentation
Standard Operating Procedures (SOPs)
SOPs outline standardized steps for performing tasks and processes. They ensure consistency and compliance with regulatory requirements, serving as the foundation for documentation practices.
Batch Records
Batch records capture the entire manufacturing process, including raw materials, equipment, and test results. These records are vital for ensuring product consistency and demonstrating regulatory compliance.
Quality Control Documents
Quality control records document testing outcomes and investigations, supporting the integrity of products throughout their lifecycle. They are critical for audits, traceability, and maintaining high-quality standards.
Partner with Our Experts
Gain clarity on implementing Current Good Documentation Practice (cGDP) in your operations. RookQS is ready to help you achieve compliance and enhance efficiency. Connect with Us Today!
