What Is Design Verification?
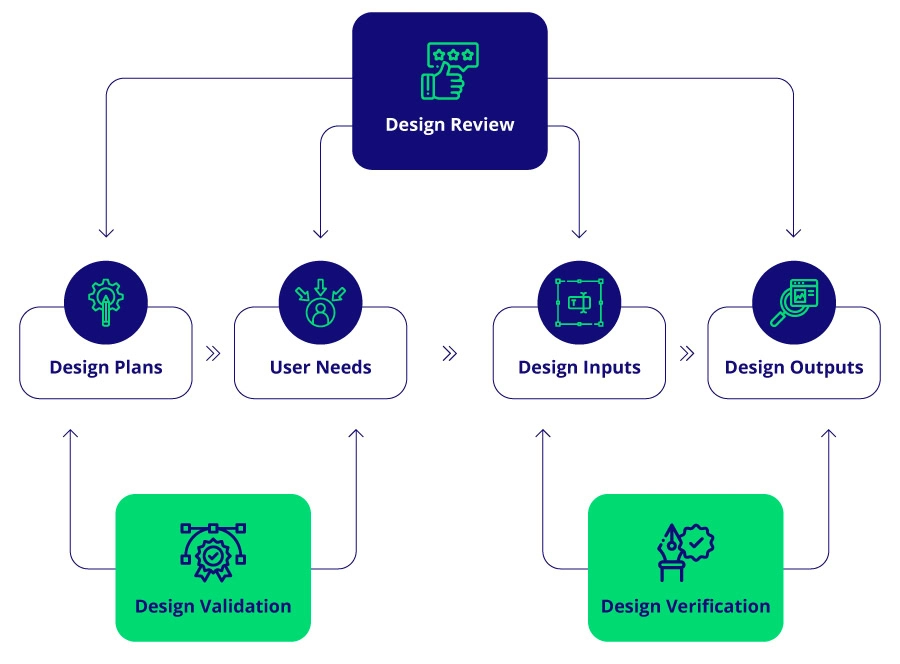
Design verification is a rigorous process that confirms whether a medical device’s design outputs meet all specified requirements, including regulatory standards, ensuring the product performs as intended. It verifies alignment between the device’s design and the initial specifications or user needs, answering the critical question, “Did we design the device right?”
The primary objective of design verification is to provide objective evidence that the design outputs satisfy their corresponding design inputs throughout the entire design space. This process often includes testing under “worst-case” conditions to assess the adequacy of the design in meeting all performance and safety requirements. In some instances, manufacturers may justify reducing sample sizes for design verification testing if there is a robust plan for process validation and 100% inspection of critical characteristics.
Design Verification Process
The design verification process involves a structured set of activities that confirm, through examination and documented evidence, that the product design meets all requirements for the intended user. This process relies on thoroughly understanding user needs and establishing clear, appropriate design inputs.
Key activities in design verification include:
- Inspections, Testing, and Analysis: Objective measurements and testing validate that design outputs conform to design inputs.
- Demonstrations: Physical or simulated demonstrations may show performance under various use conditions.
- Documentation: Carefully document the evidence to demonstrate that the requirements have been met.
Complex devices often require a variety of verification activities, from thermal analysis to package integrity testing. For medical devices, verification confirms that design outputs align with inputs, ensuring the device’s safety and effectiveness.
Identifying and Preparing
Successful design verification hinges on establishing precise design inputs that accurately reflect user needs, which is foundational in generating relevant design outputs.
According to FDA 21 CF2 820.30(f), verification involves “confirmation by examination and provision of objective evidence” that requirements are met. Sample sizes used in verification must be statistically justified; testing must sufficiently represent the design space, including worst-case scenarios when full coverage isn’t feasible.
Verification activities typically extend beyond product testing, encompassing examinations to ensure compliance with regulatory requirements and specifications documented in the design plans.
Planning
A successful verification process is rooted in a strong design plan that clearly understands user needs. A verification plan should be developed with design inputs, ensuring all requirements are addressed across the design process.
Critical Components of a Design Verification Plan:
- Types of Testing: Common tests include thermal analysis, failure mode analysis, biocompatibility testing, and others tailored to the device’s intended use.
- Acceptance Criteria: Criteria must be established before verification begins to ensure consistency and rigor.
- Differentiation Between Verification and Validation: Verification ensures the device meets technical specifications, while validation confirms the device fulfills user needs.
This planning phase ensures the alignment of design verification activities with the broader goals of regulatory compliance and device safety.
Developing Protocols
In the product development phase, protocols for design verification are crafted to ensure comprehensive testing and tracking. This phase involves developing, executing, and documenting test cases for each design input, often using methodologies like Scrum or Waterfall for structured development.
Essential Activities in Development:
- Traceability Matrix: This document links design inputs with corresponding tests, ensuring complete coverage.
- Change Control: Documenting and managing design inputs or output modifications ensures quality and compliance.
- Documentation System: Organized documentation streamlines the development process, supporting regulatory compliance and easing the pathway to market.
Executing
The execution phase implements test procedures defined in the verification plan, verifying that design outputs meet the specified design inputs.
Execution Steps:
- Test Procedure Execution: Testing follows the predetermined plan, with any deviations or invalid results documented, reviewed, and managed as defects.
- Defect Resolution: Upon resolution, regression testing confirms no new issues have been introduced.
- Traceability Matrix Updates: This ensures that all requirements have been tested and passed.
Reporting
Thorough reporting is essential for documenting the design verification process. Report record test configurations and results and identify issues at each phase.
Key reporting activities include:
- Detailed Verification Reports: Document configuration management, testing outcomes, and product version information to capture all verification aspects.
- Traceability Reports: Link verification results back to design inputs, ensuring transparency and comprehensive coverage.
- Review and Approval: Verification reports undergo review and approval, reinforcing an iterative quality assurance process.
Best Practices for Design Verification
- Integration with Quality Management Systems (QMS): Design verification should be an integral part of the QMS to support audit readiness and robust risk management.
- Use of Digital Tools: Tools like electronic data capture (EDC) can streamline verification activities, enhancing data accuracy.
- Ongoing Assessments: Regular assessments confirm that design specifications are met, supporting long-term quality management.
Validation vs. Verification
The distinction between verification and validation is crucial:
- Design Verification confirms that the design outputs meet design inputs, ensuring the device was created correctly.
- Design Validation proves that the device fulfills user needs and intended uses, verifying that the correct device was developed.
In short, verification is an internal check of specification alignment, while validation is an external check of user needs fulfillment. Effective design verification and validation processes are complementary; a device that passes verification may still require validation to ensure real-world efficacy.
Medical Device Regulatory Requirements
Medical device manufacturers must comply with the FDA’s Design Control Guidance (FDA 21 CFR 820.30(f)). This includes:
- Sample Size Justification: Ensuring statistically valid results.
- Comprehensive Verification Plan: Outlining objectives, test methods, acceptance criteria, and detailed documentation.
- Validation on Representative Prototypes: Ensuring that prototypes used in validation reflect the final design to yield applicable results.
FDA also mandates validation activities such as clinical evaluations and usability testing to confirm that devices achieve intended outcomes in real-world settings.
Ensuring Compliance and Reliability Through Effective Design Verification
Design verification is critical in medical device development, confirming that each design output aligns with specified requirements before regulatory submission. Rigorous testing, structured protocols, and adherence to FDA guidelines ensure that the device meets regulatory standards and user needs. By prioritizing thorough documentation and systematic verification, manufacturers streamline the path to market while ensuring safety, reliability, and lasting quality in the field.
Streamline Your Design Verification Process
Ready to ensure your medical device meets all regulatory requirements and performs as designed? At Rook Quality Systems, we specialize in guiding medical device companies through the complexities of design verification. Schedule a consultation with our team to discuss how we can help you streamline your verification process, strengthen compliance, and expedite your path to market. Let’s get started—reach out today to build a solid foundation for success.
