Quality Management Review (QMR)
A Quality Management Review (QMR) ensures ongoing medical device compliance by evaluating the performance and effectiveness of the quality management system, which is crucial for meeting regulatory standards. These reviews evaluate past performance and identify areas for continuous improvement, helping organizations remain compliant while enhancing their market position.
What is a Quality Management Review?
A Quality Management Review systematically evaluates the performance of the company’s quality management system (QMS). Its primary purpose is identifying improvement opportunities while ensuring alignment with the organization’s strategic direction. QMRs are essential for maintaining regulatory compliance, particularly in industries like medical device manufacturing, where adherence to standards such as ISO and FDA regulations is critical.
This review typically includes evaluating how well the quality management system meets the company’s goals and objectives, documenting actions, and assessing whether current processes effectively drive continuous improvement. By documenting every step, the QMR helps medical device companies ensure that their QMS is compliant and optimized for performance.
Why Are Management Reviews Important?
Management reviews are essential for maintaining regulatory compliance and improving product quality, safety, and efficacy in medical device manufacturing. These reviews are a required component of many international standards, including ISO 13485:2016 and FDA 21 CFR Part 820, which govern quality systems in the medical device industry.
Beyond compliance, QMRs offer strategic benefits by continually improving the quality system and addressing potential quality issues before they impact production or product safety. Regular management reviews lead to increased efficiency, reduced risks, and better resource allocation, enhancing the overall effectiveness of the organization’s quality system.
Management Responsibility
Top management ensures that Quality Management Reviews are conducted effectively and meet regulatory and strategic objectives. Management is responsible for setting the tone of the organization’s quality culture and allocating resources to address identified quality issues or opportunities.
Failure to properly conduct and act upon QMRs can have significant consequences, including FDA warning letters, recalls, or even product market withdrawal. Management’s commitment to quality, risk mitigation, and compliance audits is key to long-term success in the medical device industry.
How Often Should a Management Review Occur?
The FDA requires management reviews to be conducted annually to assess the quality system’s effectiveness and fitness. However, it is recommended that management reviews be performed more frequently, such as quarterly or at least twice a year, based on the organization’s size, the number of issues experienced, organizational changes/periods of transformation, or the timeframes of response to critical issues.
Some critical metrics, such as non-conformances and customer feedback, should be assessed monthly. For less critical items, quarterly reviews may suffice. The frequency of QMRs should be tailored to the organization’s size, complexity, and risk profile.
What Are the Regulatory Requirements?
Several key regulatory frameworks and standards govern quality management reviews for medical device manufacturers. These regulations ensure that QMRs are integrated into quality management practices and necessary for ongoing compliance.
Key Regulatory Standards:
- ISO 13485:2016 – Specific to medical device manufacturers, outlining how quality management systems should be implemented.
- FDA 21 CFR Part 820 – Governs quality system regulations for finished medical devices intended for human use.
FDA 21 CFR Part 820
FDA 21 CFR Part 820 sets forth the regulations for medical devices to ensure that the finished product meets regulatory requirements and the manufacturer’s quality objectives. A key part of these regulations is the requirement for regular Quality Management Reviews to evaluate and document the effectiveness of the quality system.
The goal is to ensure that all quality systems, including those related to production and process controls, design, and corrective and preventive actions (CAPA), meet FDA standards.
ISO 13485:2016
ISO 13485:2016 is the internationally recognized standard for quality management systems specific to medical device manufacturers. It emphasizes risk management, process control, and the documentation of quality management system processes.
The main difference between ISO 13485:2016 and other quality standards is its focus on medical devices. Adoption of this standard is crucial for regulatory compliance. Certification involves rigorous audits, and regular management reviews are essential for maintaining it.
What to Include in a Management Review
A successful management review relies on various inputs, outputs, and supporting documents, all of which must be adequately documented (agenda, meeting minutes/signatures of attendees). Each component plays a crucial role in determining the effectiveness of the quality management system and driving continuous improvement.
Supporting Documents
To conduct an effective review, supporting documents such as:
- Review inputs
- Corrective and Preventive Action (CAPA) reports
- Internal and external audit findings
- Customer complaints
- Quality objectives
These documents provide a comprehensive view of the quality system’s current state and highlight areas for improvement.
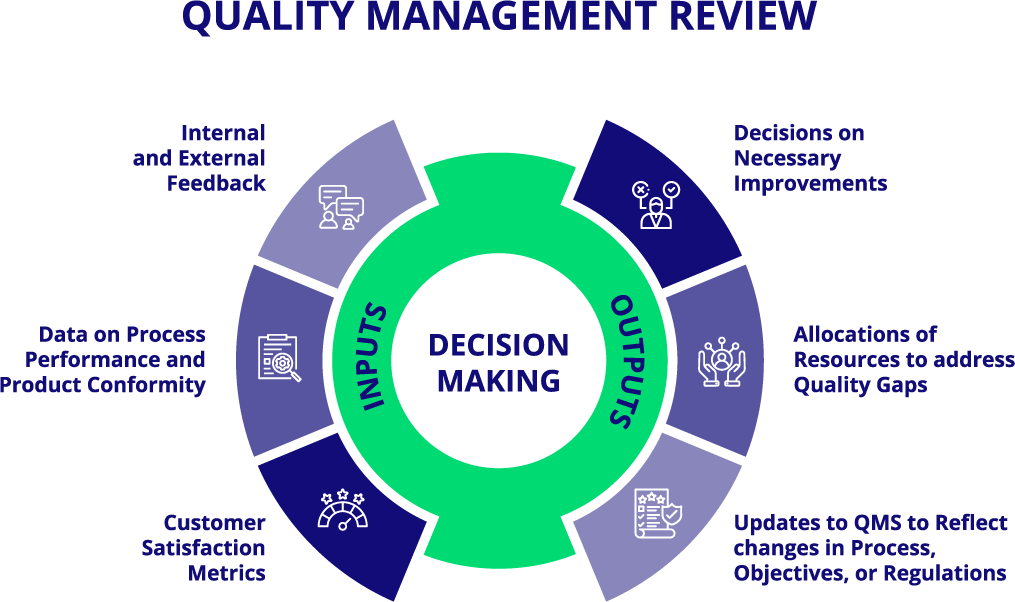
Review Inputs
Review inputs shall include, but are not limited to, the following items:
- Adequacy of Quality Policy (at least once a year during one of the Management Reviews)
- Status of Quality Objectives
- Customer Feedback
- Complaint Handling
- Reporting to Regulatory Authorities
- Process Performance
- Product Conformity
- Status of Corrective and Preventative Actions (CAPAs)
- Supplier Data
- Post-Market Surveillance Review
- Results of Audits (Internal and External)
- Follow-Up from Previous Management Reviews
- Changes in Regulatory, Competitive, Economic, and Internal Environment That Could Affect System
- Applicable New or Revised Regulatory Requirements
- Recommendations for Improvement
These inputs ensure that the review is data-driven and focuses on the most critical aspects of the quality management system.
Review Outputs
Review outputs will include any decisions or actions related to the following items:
- Improvement of the effectiveness, suitability, and adequacy of the equality management system and its processes
- Improvement of product related to customer requirements
- Response to changes in the applicable regulatory requirements
- Resource needs
These outputs provide the foundation for continuous improvement and ensure the quality management system evolves alongside the business.
Would You Like to Know More About Quality Management?
At Rook Quality Systems, we are experts in implementing and maintaining quality management systems tailored to the medical device industry. Our team helps companies optimize their QMS, ensuring full regulatory compliance and improving product quality.
Streamline with RookQS
RookQS offers expert support in streamlining quality management reviews by guiding resource allocation, risk management, and compliance with ISO standards. Our solutions ensure that your management review process is efficient and aligned with your business objectives.
Contact RookQS today to explore our comprehensive services and learn how we can help you optimize your quality management system.