Quality Management System (QMS)
What Is a Quality Management System (QMS)?
A Quality Management System (QMS) is a structured framework that documents processes, procedures, and responsibilities for achieving quality objectives. The main goal of a QMS is to ensure that products and services consistently meet customer expectations and regulatory standards, like ISO 13485 and ISO 9001.
For industries such as medical devices, implementing QMS software helps automate critical tasks like auditing, compliance monitoring, and staff training, reducing manual workflows and boosting efficiency. This automation supports continuous improvement and ensures product or service quality at every stage of development and production.
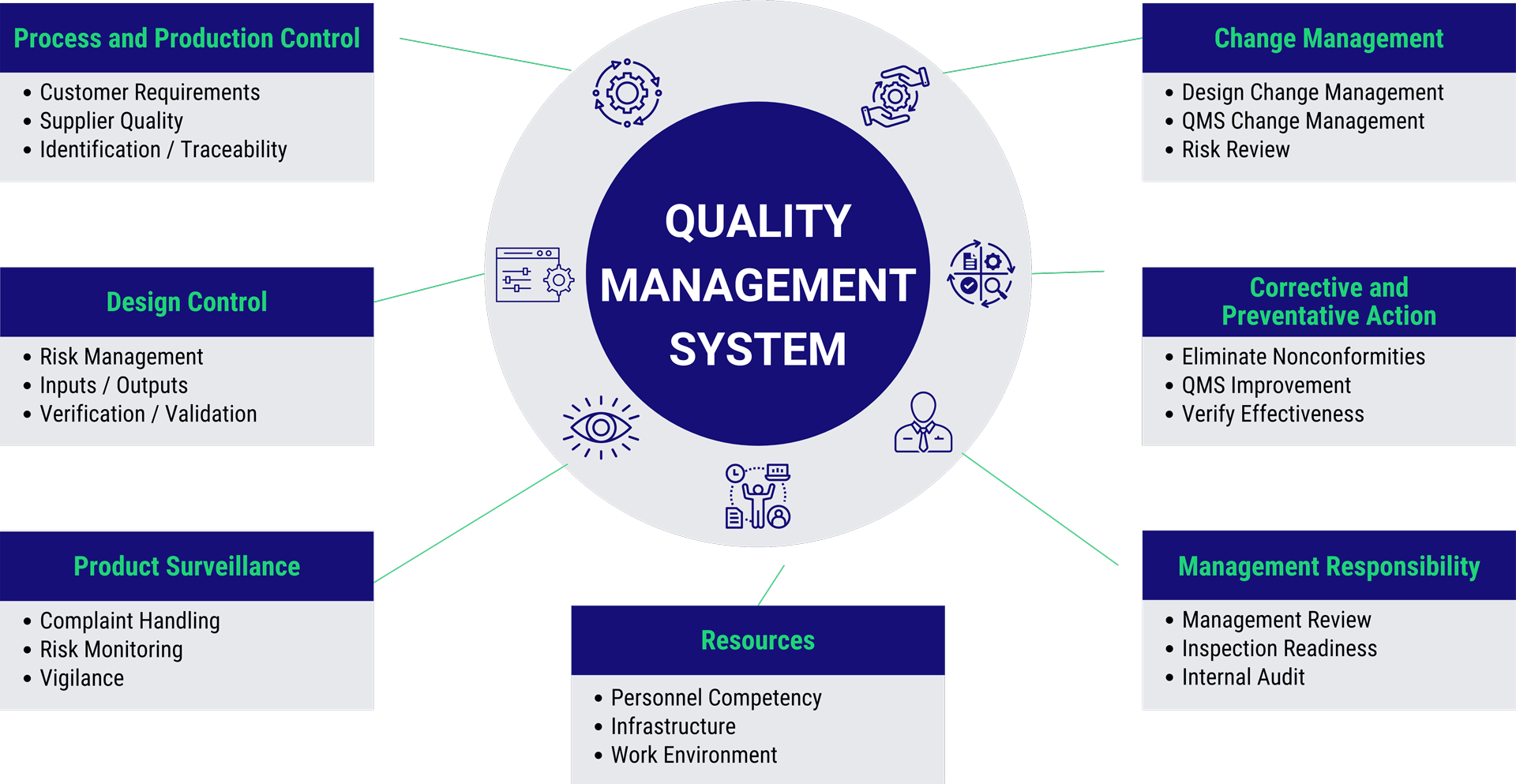
Why is a QMS Essential?
A QMS is crucial for organizations to consistently meet customer requirements and comply with regulatory standards. It promotes a culture of continuous improvement and brings several key benefits:
Traceability
A QMS ensures that products and processes are fully traceable from design to delivery. This traceability is vital for compliance and managing product recalls, simplifying root cause analysis when issues arise.
Compliance
By adhering to global standards like ISO 13485 and FDA 21 CFR 820, a QMS ensures that products meet the stringent safety and quality requirements set by regulatory bodies, minimizing the risk of non-compliance.
Benefits to End Users
A well-maintained QMS ensures that customers receive safe, reliable, and effective products, leading to higher satisfaction and trust. Quality products foster long-term customer relationships and build brand loyalty.
Increased Company Value
An established QMS demonstrates a company’s commitment to quality and operational excellence, increasing the organization’s market value for investors, potential buyers, and clients.
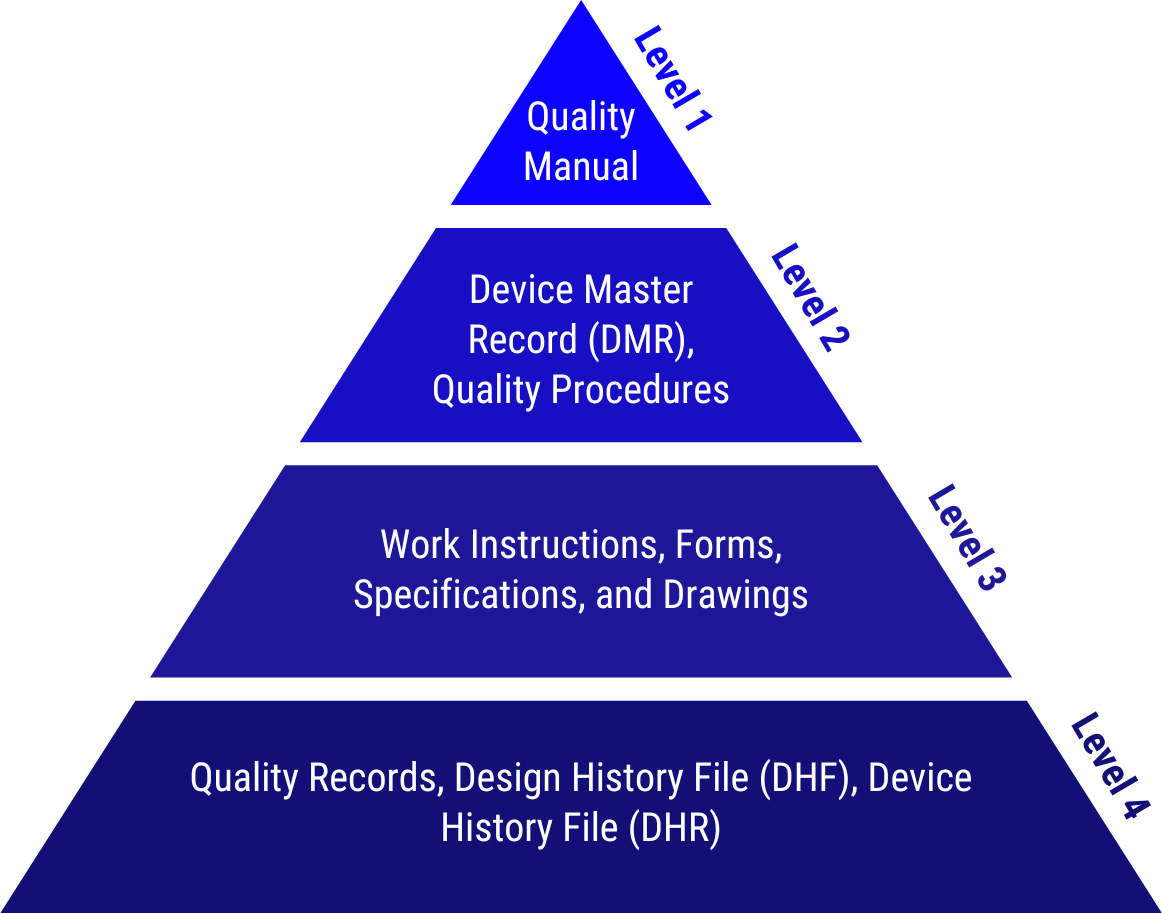
Elements of a Medical Device QMS
A Medical Device QMS ensures compliance with both regulatory and customer requirements. Its key components include:
Quality Manual
The Quality Manual defines the scope of the QMS, identifies responsible parties, and outlines key processes that ensure quality objectives are met.
Device Master Record (DMR)
The DMR contains all the information needed to manufacture a device, including drawings, specifications, and production instructions, ensuring consistency and product quality.
Design History File (DHF)
The DHF documents the design and development processes, demonstrating compliance with regulatory requirements and alignment with design inputs.
These elements work together to create an effective quality management framework that ensures the safety and efficacy of medical devices.
Policies
Policies define quality objectives and provide a framework for the QMS. These guidelines direct how the organization should operate to ensure regulatory compliance and customer satisfaction.
Standard Operating Procedures (SOPs)
SOPs are detailed instructions on how to perform specific tasks to ensure consistency, compliance, and quality throughout the production process.
Forms
Forms are templates for documenting processes and collecting data, ensuring quality management and regulatory reporting consistency.
Records
Records are a documentation repository, including production data and inspection results, demonstrating compliance with the QMS and regulatory requirements.
How is a QMS Regulated?
Global standards regulate a QMS to ensure product quality, safety, and compliance. For the medical device industry, compliance with these regulations is critical:
US Quality System Regulation (QSR) 21 CFR 820
Regulation 21 CFR 820, enforced by the FDA, requires medical device manufacturers to establish and maintain a QMS that ensures device safety and effectiveness.
ISO 13485:2016
ISO 13485:2016 is an international standard that outlines QMS requirements specifically for medical devices, focusing on risk management, regulatory compliance, and continuous improvement.
Challenges of Developing a QMS
Implementing a QMS can be challenging due to common misconceptions. Some organizations see quality control as limited to inspections, which can lead to neglect of broader quality systems and a reactive rather than proactive approach.
A key factor for successful QMS implementation is management commitment. Leadership must communicate quality policies effectively across the organization and integrate them with company goals. Organizations can overcome these challenges by aligning the QMS with customer needs and company objectives and ensuring successful implementation.
Partner with RookQS for Superior QMS Implementation
A robust QMS that complies with industry standards like ISO 13485 requires expert guidance. Rook Quality Systems specializes in custom QMS solutions that help organizations navigate complex regulatory landscapes and certification audits. Our team ensures a seamless QMS implementation process tailored to your company’s needs.
Contact us to learn how RookQS can help your organization achieve superior QMS implementation and maintain regulatory compliance.