What is Equipment Validation?
Equipment validation is the process of ensuring that a piece of equipment consistently performs its intended function according to regulatory requirements and industry standards. While the specific requirements of the validations are not dictated by regulations, performing validation for equipment that requires it is a regulatory obligation. This systematic approach involves testing and documenting each stage of the equipment validation process to confirm that the equipment operates reliably under real-world conditions. Essential in regulated industries like medical devices, pharmaceuticals, and biotechnology, equipment validation safeguards product quality, patient safety, and compliance with FDA, ISO, and other international standards.
Why is Equipment Validation Important?
Equipment validation is vital for maintaining compliance with stringent regulatory requirements. By validating equipment to ensure it functions as intended, organizations can uphold quality assurance, produce high-quality products, and minimize risks that could impact product quality. Companies face potential issues such as production delays, product recalls, or patient harm without proper validation. Additionally, validated equipment enhances operational efficiency, reducing the need for corrective actions.
Ready to streamline your path to compliance and deliver high-quality products? Connect with our team of experts to guide you through every step of the equipment validation process.
Key Components
Equipment validation encompasses three primary stages: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each stage involves specific validation activities and follows detailed requirements to ensure the equipment’s performance meets the established acceptance criteria.
Installation Qualification (IQ)
Installation Qualification confirms that the equipment has been installed correctly per the manufacturer’s instructions. This phase includes verifying proper setup, calibration, wiring, and documentation.
Operational Qualification (OQ)
Operational Qualification tests the equipment under specified conditions to verify it operates as expected. This stage includes testing parameters such as temperature, speed, or pressure to confirm the equipment meets operational requirements. For instance, a pharmaceutical tablet press must produce tablets of consistent size and weight within defined tolerances.
Performance Qualification (PQ)
Performance Qualification ensures that the equipment delivers consistent results in real-time operational scenarios. This final validation step often involves full-scale production runs or stress tests to verify equipment performance under normal working conditions. For example, running multiple product batches using the same sterilization cycle ensures consistent sterility outcomes.
Regulatory Requirements
Equipment validation must comply with regulatory frameworks such as FDA 21 CFR Part 820 for medical devices, ISO 13485:2016 for quality management systems, and EU MDR for European markets. Guidance documents like GAMP5 provide structured methodologies for validating automated systems. Organizations must also consider regional regulations, ensuring their validation processes align with international best practices.
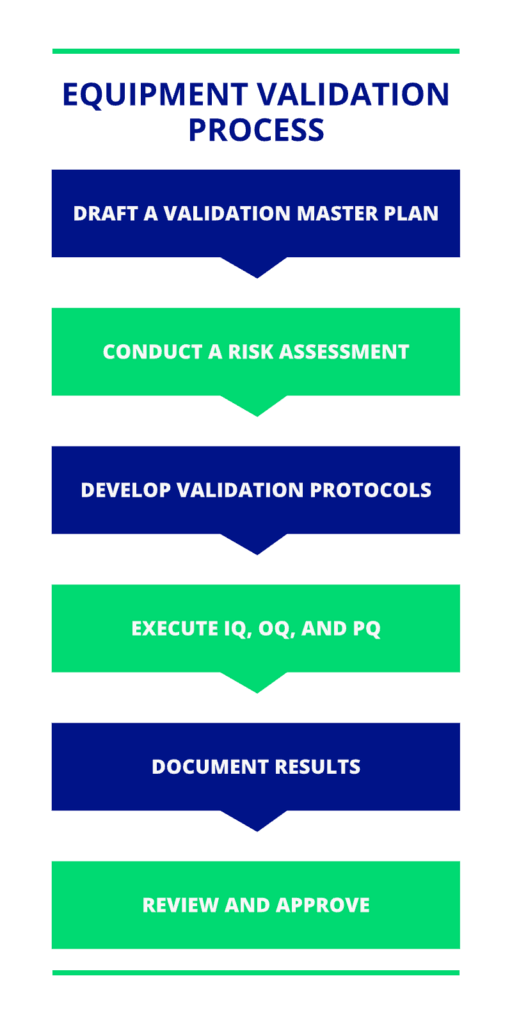
The Equipment Validation Process: Step-by-Step
The equipment validation process typically involves these steps:
- Draft a Validation Master Plan: Define the validation project’s scope, objectives, and responsibilities.
- Conduct a Risk Assessment: Identify and address risks impacting product quality or patient safety.
- Develop Validation Protocols: Outline testing procedures, acceptance criteria, and documentation requirements.
- Execute IQ, OQ, and PQ: Perform the key validation stages as outlined.
- Document Results: Maintain thorough records of validation activities, findings, and corrective actions.
- Review and Approve: Obtain formal approval from quality assurance teams and regulatory bodies.
Best Practices
To achieve success in equipment validation:
- Involve cross-functional teams: Include engineering, quality assurance, and operations to ensure comprehensive expertise and alignment.
- Follow detailed standard operating procedures (SOPs): Clearly defined SOPs for every validation activity help ensure consistency and accuracy.
- Use validation software: Leverage digital tools to automate and simplify complex documentation tasks, improving efficiency and reducing the risk of errors.
- Conduct regular reviews: Periodically assess equipment performance, protocols, and compliance to address deviations and maintain regulatory readiness promptly.
- Apply Good Documentation Practices (GDP): Ensure all records are complete, accurate, and traceable by following GDP, which supports transparency and audit readiness.
- Perform thorough risk assessments: Identify potential risks impacting product quality or safety and address them proactively during validation.
- Define clear acceptance criteria: Establish measurable standards for equipment performance to ensure validation activities meet regulatory and operational expectations.
- Train personnel effectively: Provide comprehensive training on validation protocols, regulatory requirements, and proper use of equipment to ensure all team members are equipped to execute tasks successfully.
- Maintain continuous improvement: Treat equipment validation as an ongoing process, incorporating lessons learned and updates to standards to refine practices over time.
By implementing these best practices, organizations can ensure a robust and efficient validation process, supporting compliance and operational excellence.
Additional Resources for Equipment Validation
Explore the following resources for more information:
Ensuring Validation Success
Equipment validation is a cornerstone of quality assurance in regulated industries. Organizations can streamline their equipment validation processes by leveraging tailored quality and regulatory solutions, ensuring compliance, operational efficiency, and consistent product quality.
Look to Rook
Contact us today for a consultation and ensure your equipment performs reliably, safely, and meets all regulatory requirements.
