Instruction for Use (IFU)
What are Instructions for Use (IFU)?
Instructions for Use (IFU) are detailed documents provided by manufacturers that outline the proper usage, storage, handling, and safety precautions for a product. Their primary purpose is to ensure safe and effective use while minimizing the risk of user error. IFUs are crucial in the context of labeling requirements for medical devices and prescription drug products, as they provide comprehensive guidelines that must be submitted to the Food and Drug Administration (FDA) during the pre-market approval process.
Why are IFUs Important?
IFUs play a critical role in:
- Providing comprehensive guidance to prevent human errors and hazards.
- Offering care instructions and clinical guidelines for device usage.
- Supporting training and education for healthcare professionals.
- Ensuring regulatory compliance and oversight.
- Facilitating post-market surveillance and ongoing compliance.
Regulatory Guidelines for IFUs
The FDA has stringent guidelines for IFUs, ensuring they include all necessary information for safe and effective product use. This includes device descriptions, instructions for use, warnings, and compliance information. IFUs must be kept up-to-date and reviewed regularly to comply with regulatory standards. In accordance with Title 21 of the Code of Federal Regulations, most medical equipment, devices, and accessories intended for clinical use must include an IFU, though there are certain exceptions.
Components of an Effective IFU
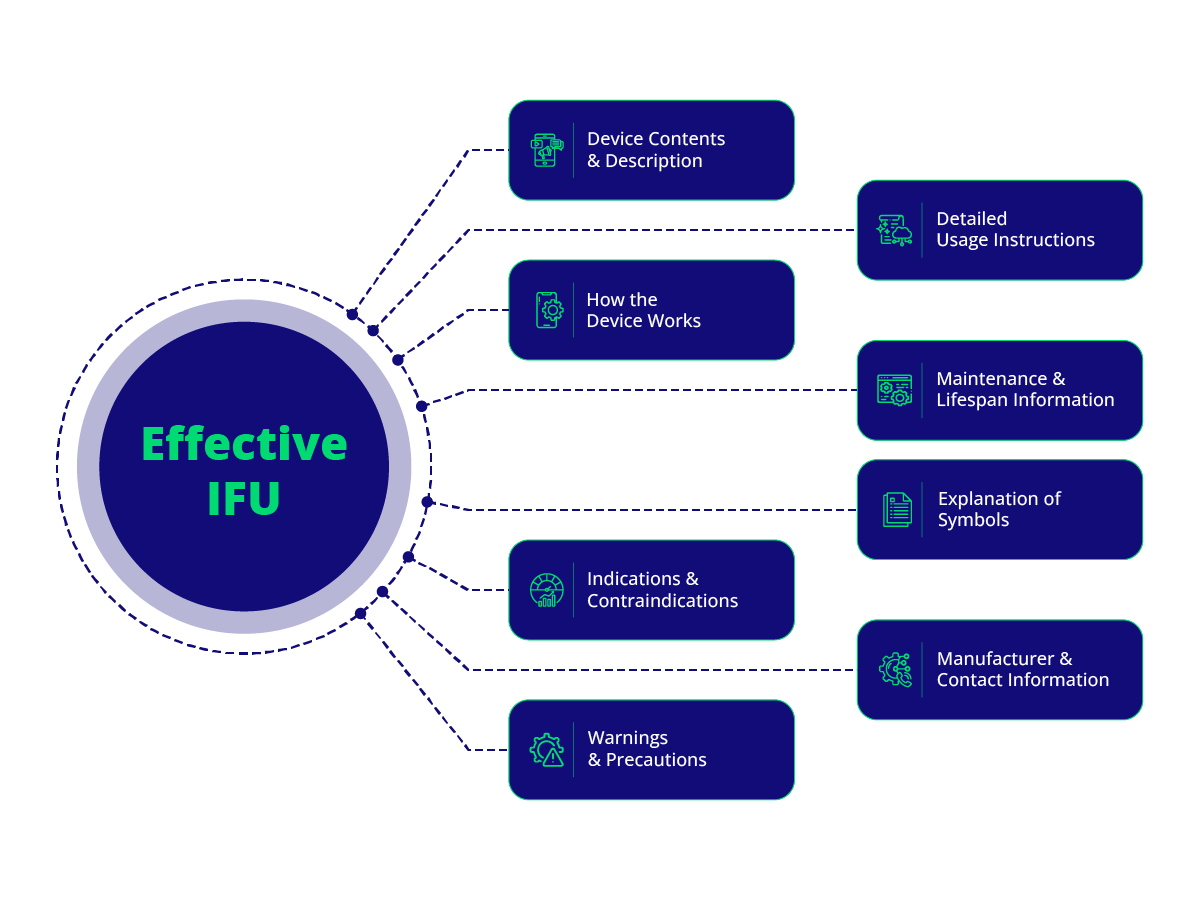
An effective IFU typically includes:
- Contents and description of the device.
- How the device works.
- Indications and contraindications for use.
- Warnings and precautions.
- Detailed usage instructions, including steps for operation, adjustment, cleaning, and storage.
- Maintenance and lifespan information.
- Explanation of symbols used in the documentation.
- Manufacturer and contact information.
Examples of IFUs
To see a comprehensive IFU, refer to the Zimmer Biomet Vanguard Total Knee Surgical Technique.
Best Practices for Maintaining IFUs
Annual Review: Integrate a yearly review of the IFU within the management review process to ensure it remains up-to-date and compliant with regulatory standards.
Timely Updates: Revise the IFU immediately when new information or updates about the device are available, ensuring users have the most current and accurate instructions.
For more detailed guidelines, refer to the FDA’s guidance on medical device labeling.
Learn More About Instructions for Use (IFU)
Discover how Rook Quality Systems, your Medical Device Quality Consultants, can assist in creating and maintaining effective IFUs.