Notified Bodies
What is a Notified Body?
A Notified Body is an independent organization designated by an EU country to assess the conformity of certain products, including medical devices, before entering the European market. Their primary function is to ensure that products meet the requirements of EU directives and regulations requirements, like the Medical Devices Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), before market release.
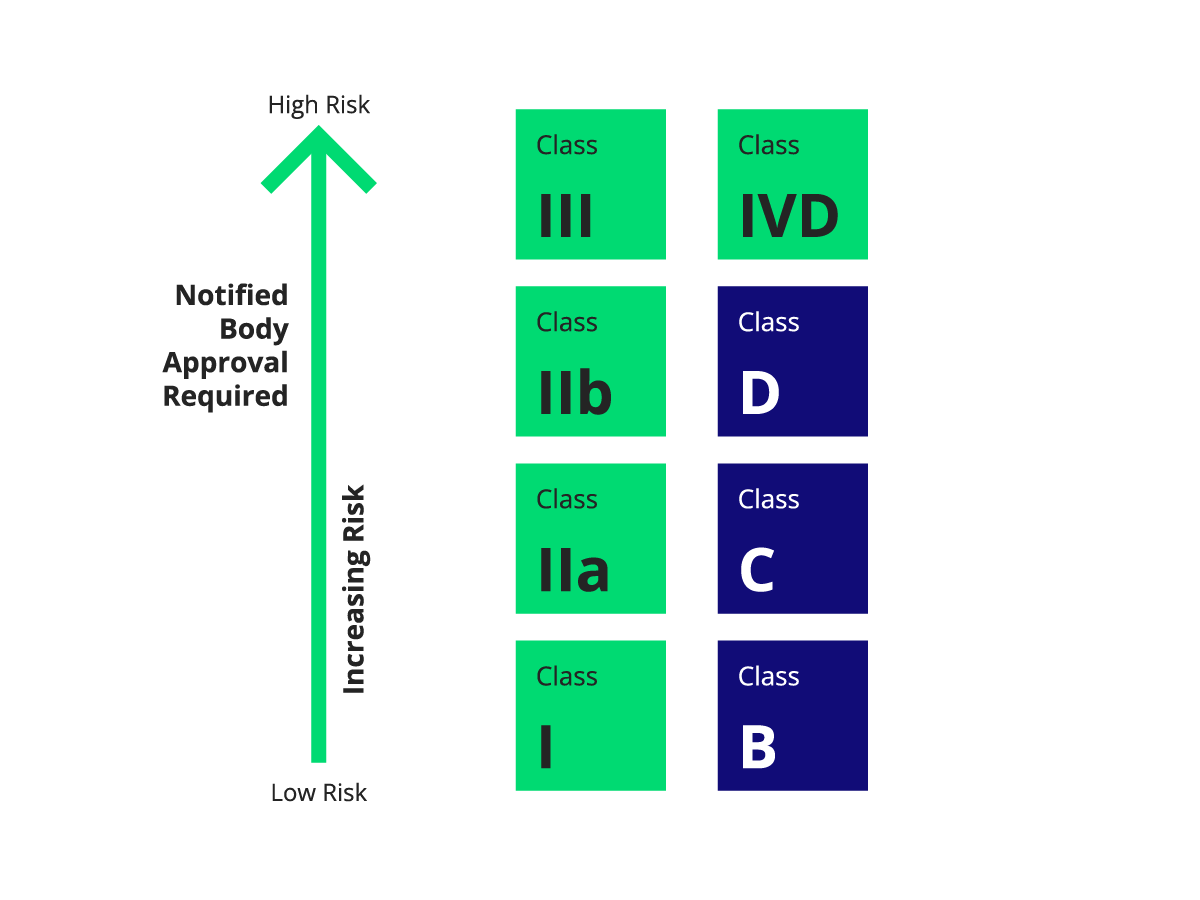
For medical devices, Notified Bodies play a crucial role in certifying higher-risk devices, such as Class II and III devices or in vitro diagnostic devices (IVDs), confirming that these products comply with the required safety and performance standards. Manufacturers of these products must obtain certification from a Notified Body to label their devices with the CE Mark, allowing them to be sold in the EU market.
Why Are Notified Bodies Important?
Notified Bodies ensure that only safe, compliant products are allowed onto the EU market. They play an essential role in:
- Ensuring Product Safety: Conducting thorough conformity assessments protects consumers from unsafe products.
- Facilitating Market Access: By certifying that devices meet EU standards, manufacturers can sell their products across the European Economic Area.
- Supporting Regulatory Compliance: Helping manufacturers navigate the complex landscape of MDR/IVDR, ensuring that devices meet all regulatory requirements for safety and performance.
Without NB’s involvement, manufacturers could face significant delays or even denial of market access, which could impact product success and expansion.
Key Roles and Responsibilities of Notified Bodies in Medical Devices
Notified Bodies serve as essential gatekeepers in the medical device industry, working closely with manufacturers to ensure compliance through several activities:
Conformity Assessment for High-Risk Devices
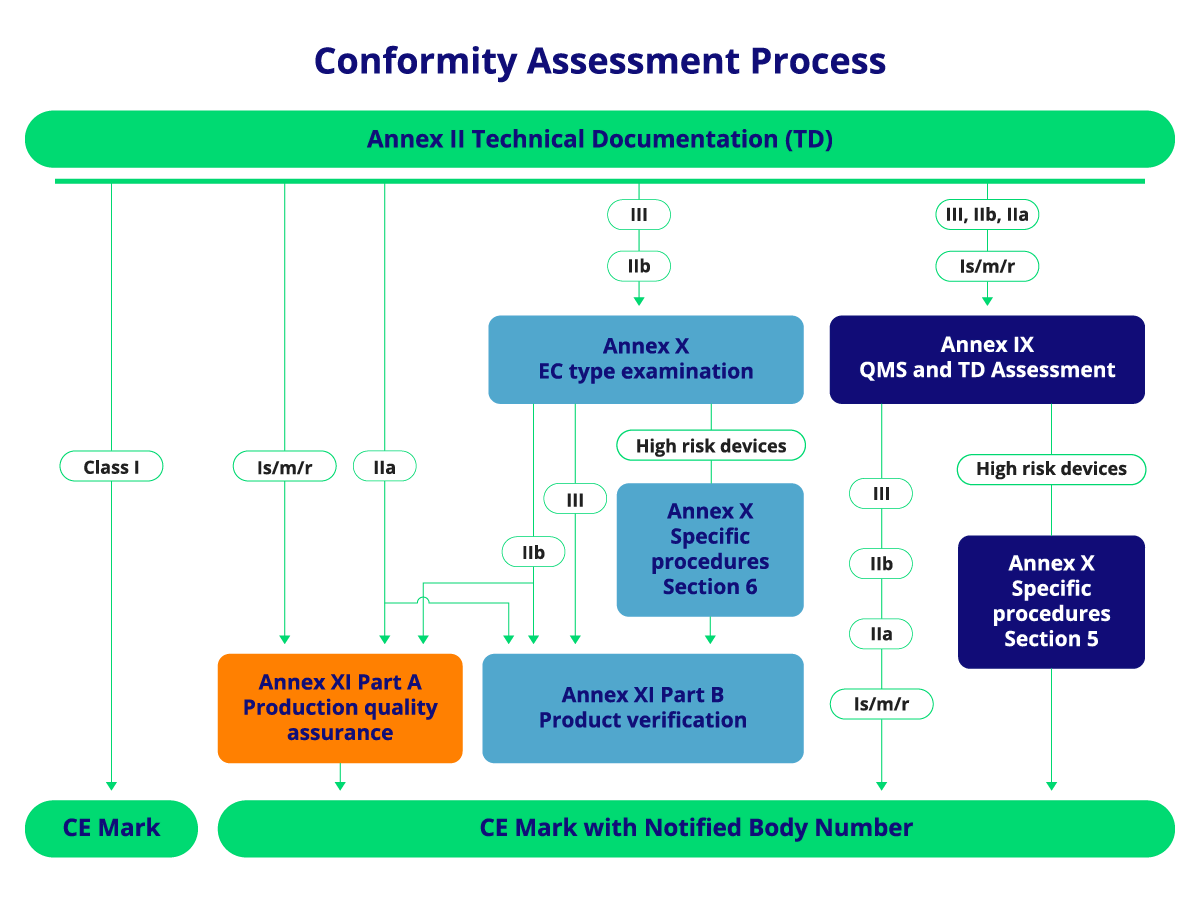
Conformity assessment is the systematic process of evaluating whether a medical device meets the applicable regulatory standards. Notified Bodies conduct assessments for devices classified as high-risk, including Class IIa, IIb, and III medical devices, as well as Class B, C, and D IVDs. They review technical documentation, risk management processes, and clinical evaluations to ensure the device’s safety and performance.
Manufacturers of lower-risk Class I devices may not require a Notified Body for conformity assessment unless their products are sterile, have measuring functions, or are reusable surgical instruments.
Device Types Requiring Notified Body Involvement
Notified Bodies must be involved in the conformity assessment process for devices classified as:
- Class IIa, IIb, and III medical devices.
- Certain Class I medical devices (e.g., sterile devices, those with measuring functions, reusable surgical instruments).
- Class B, C, and D in vitro diagnostic devices (IVDs).
Expectations for Device Manufacturers
When working with a notified body, manufacturers must fully comply with the MDR/IVDR requirements. They are expected to provide detailed technical documentation, maintain post-market surveillance programs, and conduct risk management activities. These processes must be well-integrated to facilitate a smooth conformity assessment.
Effective communication with the Notified Body is key to ensuring timely certification. This involves clear documentation, regular audits, and adherence to harmonized standards. Manufacturers should also conduct gap analyses to meet the latest regulatory updates.
CE Marking and the Role of Notified Bodies
The CE Mark signifies a manufacturer’s declaration that their device meets all relevant EU regulations, particularly the General Safety and Performance Requirements (GSPR) under the MDR. Notified Bodies certify products before they can carry the CE Mark, especially in higher-risk categories.
Notified Bodies evaluate the device’s design, quality systems, and technical documentation to ensure compliance with these requirements. The CE Mark allows products to be marketed freely across the EU, symbolizing safety and regulatory compliance.
How to Select a Notified Body
Choosing the right Notified Body is a crucial step for manufacturers. Key considerations include:
- Designation for Specific Device Categories: Ensure the Notified Body is accredited for your device’s category and the type of conformity assessment required.
- Reputation and Expertise: Research the Notified Body’s experience with similar products and their reputation for timely and efficient assessments.
- Communication and Support: Evaluate how the Notified Body aligns with your company’s communication style and operational timelines. A collaborative relationship can streamline the audit process and minimize delays.
NANDO Database
The NANDO (New Approach Notified and Designated Organisations) database provides verified information about Notified Bodies, including their designation and tasks under MDR and IVDR. Manufacturers can use this tool to identify Notified Bodies accredited for their specific device category and track updates on their certification status.
NANDO for MDR
Manufacturers of medical devices can search the NANDO database for Notified Bodies designated for MDR conformity assessments. This is essential for ensuring compliance with the updated EU regulations that replaced the older Medical Device Directive (MDD).
NANDO for IVDR
The NANDO database is equally important for manufacturers of in vitro diagnostic devices seeking certification under the IVDR. It provides a reliable list of Notified Bodies authorized to conduct conformity assessments for various IVD categories.
Simplify Conformity Assessments with Expert Support from RookQS
Navigating the conformity assessment process can be daunting, but RookQS offers a streamlined solution. Our tailored Quality Management System (QMS) ensures that your documentation, risk management, and post-market surveillance are audit-ready. Whether preparing for MDR or IVDR compliance, RookQS can help you:
- Organize technical documentation.
- Ensure that clinical evaluations and risk management activities are aligned with regulatory expectations.
- Smoothly navigate Notified Body audits, reducing the risk of non-conformances.
Contact us today to learn more about how we can support your regulatory and quality needs.