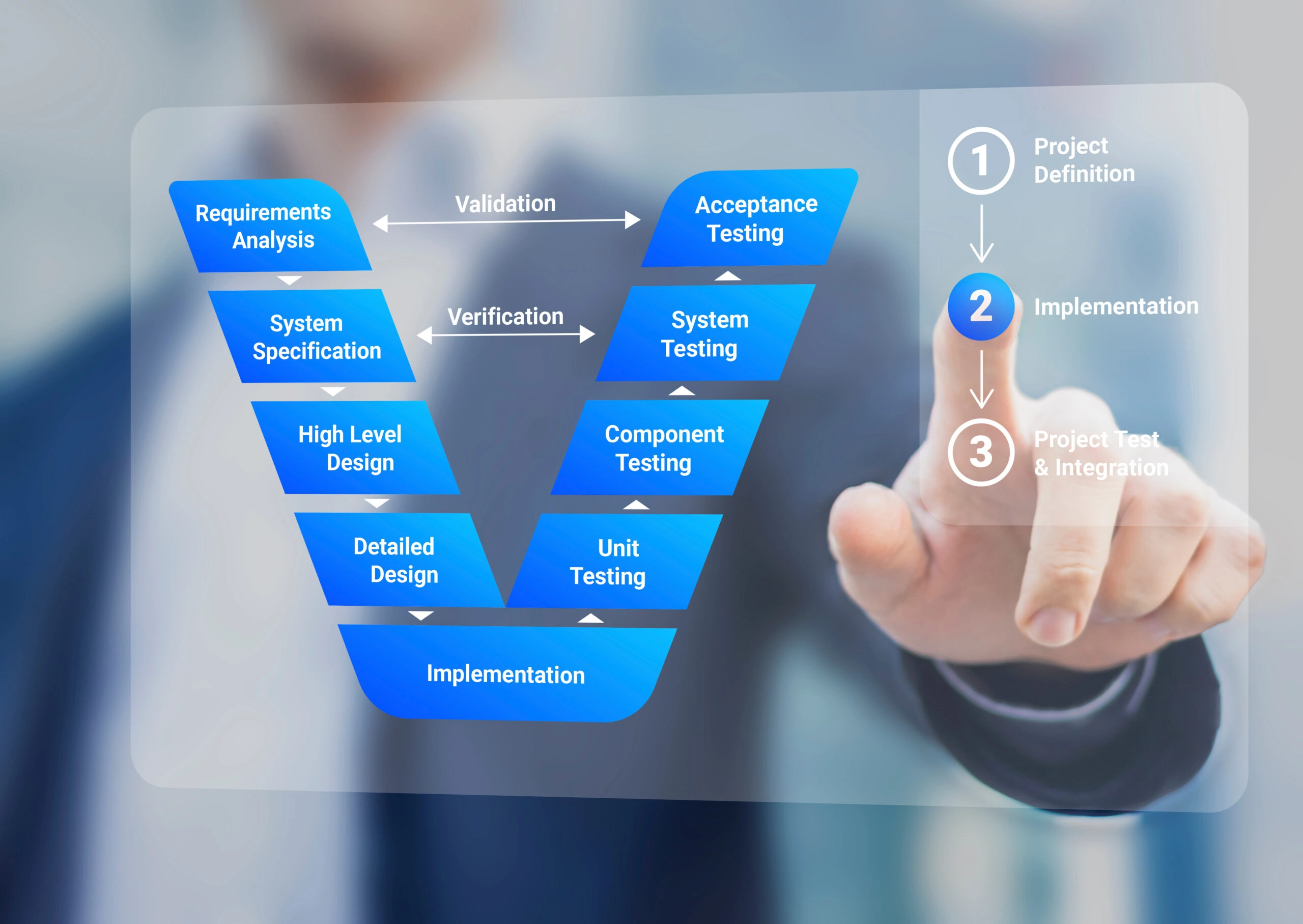
Keeping Up with the Machine: Predetermined Change Control Plans for Medical Devices
Machine learning (ML) revolutionizes the medical field by enabling faster disease detection, more accurate diagnoses, and personalized treatments. These advancements rely heavily on the vast amounts…