From Pandemic to Endemic: What Should Medical Device Manufacturers Do for COVID-19 Transition?
The COVID-19 pandemic has had a significant impact on the medical device industry. During this time, many medical devices became commercially available under enforcement policies, and plenty of medical device manufacturers have received emergency use authorization (EUA) as well. As the public health emergency wanes off and the COVID-19 pandemic is transitioning to COVID-19 endemic, it is essential for medical device manufacturers to pay close attention to FDA’s instructions on EUA transition activities.
FDA COVID-19 Transition Guidances:
- Transition Plan for Medical Devices That Fall Within Enforcement Policies Issued During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency
- Transition Plan for Medical Devices Issued Emergency Use Authorizations (EUAs) Related to Coronavirus Disease 2019 (COVID-19)
The RookQS team developed this blog to explain who is subjected to this EUA transition, when the manufacturers should start the process, and the quality and regulatory considerations you should be fully aware of. RQS is confident that our clients will be able to develop their own customized COVID-19 transition plan smoothly and effectively with our guidance.
Who should care about EUA transition?
Manufacturers who have received EUA or whose devices are subjected to enforcement policies are required to complete the transition process, regardless of their intention on continuing distribution. The manufacturers mentioned in the table are in the scope of FDA COVID-19 transition guidance:
| Intend to Continue Distribution | NOT Intend to Continue Distribution | Required to Discontinue Distribution | |
|---|---|---|---|
| EUA Device | O | O | O |
| Enforcement Policy Device | O | O | O |
EUA Devices
HHS issued 4 EUA declarations, which have not been terminated:
In vitro diagnostics, Personal respiratory protective devices, Medical devices and alternative products used as medical devices, and Drugs and biological products.
Enforcement Policy Device
Remote Digital Pathology Devices, Imaging Systems, Non-Invasive Fetal and Maternal Monitoring Devices Used to Support Patient Monitoring, Telethermographic Systems, Digital Health Devices for Treating Psychiatric Disorders, Extracorporeal Membrane Oxygenation and Cardiopulmonary Bypass Devices, Remote Ophthalmic Assessment and Monitoring Devices, Infusion Pumps and Accessories, Face Shields, Surgical Masks, and Respirators, Gowns, Other Apparel, and Gloves, Sterilizers, Disinfectant Devices, and Air Purifier, Ventilators and Accessories and Other Respiratory Devices, Modifications to FDA Cleared Molecular Influenza and RSV Tests, Measurement of Viscoelastic Properties: Enforcement Policy, and Viral Transport Media.
When should I get started with EUA transition?
EUA and enforcement policy devices will no longer be authorized for use in the US market after the termination date. Please note that this termination date is not dependent on an HHS PHE declaration under the PHS Act, therefore an EUA may remain authorized and new EUAs may continue being issued as long as the applicable EUA declaration and determination remains in effect. Nonetheless, FDA highly encouraged the manufacturers to be proactive on the transition activities (e.g., even before the start of the 180-day transition period), to help avoid disruption in device supply and help facilitate compliance with applicable FD&C Act requirements.
For EUA device manufacturers

- EUAs remain in effect until the relevant EUA declaration under section 564 of the FD&C Act is terminated or FDA otherwise revokes a specific EUA.
- An advance notice of termination of each EUA device will be published 180 days before the EUA termination date.
- During the 180-Day transition period, manufacturers shall complete several transition activities (e.g., transition implementation plan, agency review on the aforementioned plan) and must continue to comply with the terms of the devices’ respective EUAs.
- The device will no longer be authorized for use beginning on the EUA termination date.
For enforcement policy device manufacturers

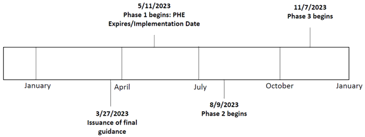
- The PHE declaration expired on May 11, 2023.
- During the 180-Day transition period, manufacturers shall complete several transition activities (e.g., submit a marketing submission and it is accepted before the EUA termination) and must continue to comply with the enforcement policy.
- The enforcement policy is no longer in effect on November 7, 2023, and the devices will no longer be authorized for use.
In order to continue the distribution of devices and always keep one step ahead of the competitors, the manufacturers need to promptly start preparing a marketing submission, and there are some key aspects that should be considered for this transition.
Here are the five key considerations for COVID-19 transition.
Safety and Effectiveness of Devices
Safety and effectiveness of the device must be prioritized. It is essential to ensure that the submitting versions of devices which will replace the EUA and enforcement policy version are verified and validated, and are able to provide accurate and reliable results. Manufacturers may need to conduct clinical studies or performance testing to demonstrate the safety and effectiveness of the devices.
Regulatory Requirements
Manufacturers need to ensure that they are complying with any applicable regulations or guidelines when transitioning away from EUA or enforcement policy devices. To this end, manufacturers have to pursue a suitable regulatory pathway and meet all the requirements of these regulations. It is also important to outline the documentation and data needed for marketing authorization, as well as the timelines and deadlines for marketing submission.
Quality Requirements
Manufacturers shall review and update the quality management system. It is crucial to review the existing QMS to ensure that it covers all necessary aspects related to the COVID-19 transition, and the QMS should be updated to reflect any changes, or any new risks associated with the transition process.
Supply Chain Considerations
Supply chain impacts are also vital for COVID-19 transition. Manufacturers shall ensure that there is adequate supply of the devices and that they can ramp up production and distribution to meet the demand, meanwhile ensuring the quality of the devices are consistent with the marketed devices.
Stakeholders Communication
Communication with stakeholders is essential during the COVID-19 transition process. Manufacturers should collaborate with device distributors, healthcare facilities, healthcare providers, patients, and consumers, as appropriate, to assist all stakeholders with transition planning. It is crucial to provide clear and accurate information about the safety and effectiveness of devices, as well as any changes in the availability or use of EUA or enforcement policy devices. Timely and transparent communication can help address any concerns or questions raised by stakeholders.
Conclusion
The COVID-19 EUA transition is an inevitable process for medical device manufacturers. Compliance with product V&V, regulatory and quality requirements, supply chain considerations, and effective stakeholder communication are all essential to ensure a smooth and effective transition. We strongly recommend medical device manufacturers to stay agile and take the immediate steps to complete the COVID-19 transition.
Still have additional inquiries?
Having difficulties with regulatory pathway assessment, consolidating premarketing submission documents (e.g., transition implementation plan, notification of intents, etc.), or gap analysis on your QMS?
RookQS’ experts can guide you through the COVID-19 transition process ensuring that you are meeting all requirements, and most importantly stay one step ahead of your competitors. Contact us today to learn more about how we can help you navigate the COVID-19 transition process!
