Shining A Light on MDR Notified Body Standard Fees
The EU Medical Device Regulations (MDR) has been a looming, dark cloud of change for Medical Device Manufacturers. Whether the discussion is focused on the new technical requirements, constantly changing deadlines, or overall cost, MDR has been at the forefront of medical device manufacturer conversations. Here at RookQS, we have analyzed the MDD to MDR transition extensively regarding Regulatory and Quality System requirements. Still, we realize a fundamental question for our clients first to consider: “Is this financially feasible?” And this question leads to conformity assessments: “How much does it cost to obtain a Notified Body?”
What is a Notified Body?
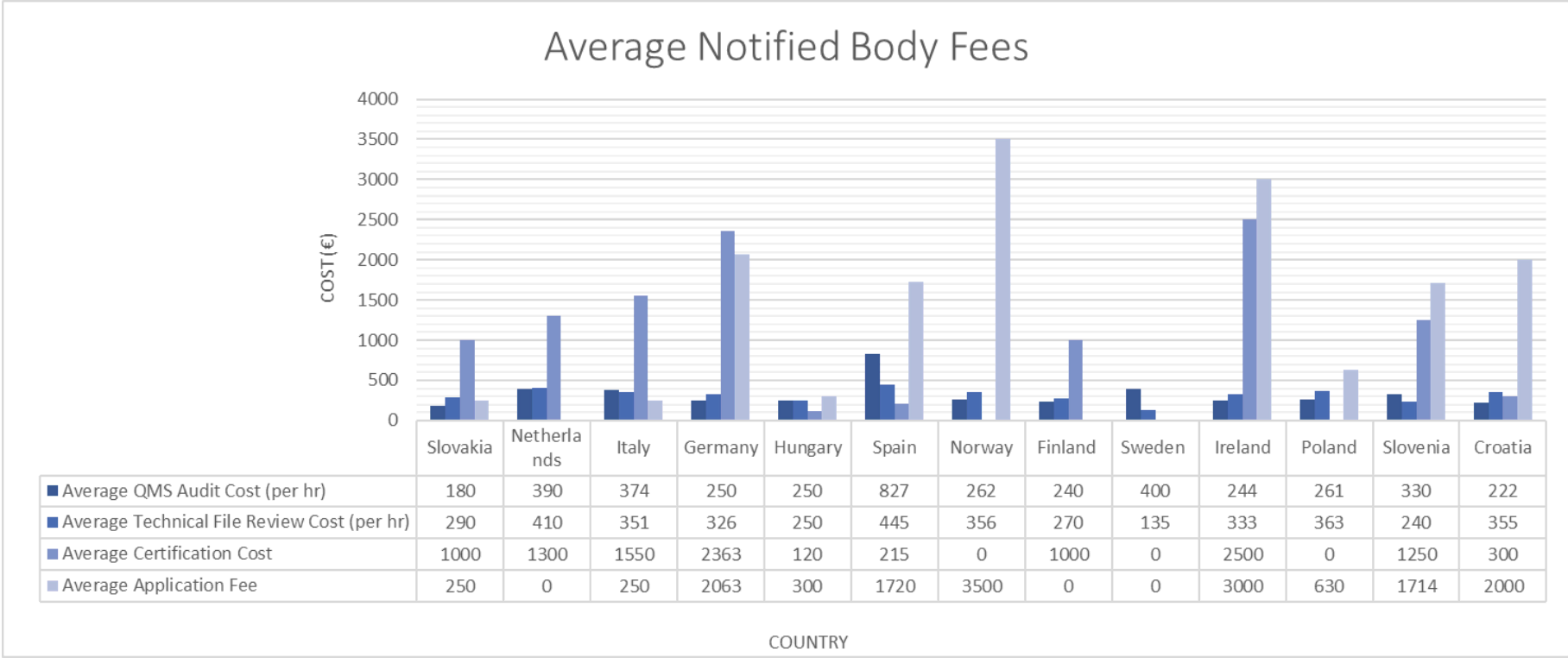
A notified body is an organization designated by an EU country to assess the conformity of certain products before being placed on the market. Did you know that EU MDR Notified Bodies must make their standard fees publicly available? The MDCG has translated the regulatory requirements further, stating that the meaning of “publicly available” implies that a member of the public can access this information at any point in time without the need for additional steps (MDCG guidance 2019-6 section V.2).
Luckily for you, we did all the legwork, acquiring 20+ lists of Standard Fees here at RookQS for the EU Notified Bodies and broke down the average application fee, hourly cost for QMS audit, hourly cost for technical file review, and certification cost across the EU countries; see Table below. We compiled the overall fees across 20+ Notified Bodies, agnostic of country. The average application fee is 1540€, the average hourly rate for QMS Audits is 325€, the average hourly rate for Technical File review is 317€, and the average certification cost is 1160€.
Our goal in gathering this data was to provide transparency and shine a light on the Notified Body pricing to help your business make financial decisions. These values do not include building your Technical File (a RookQS specialty), any testing, or traveling and unannounced audits from the Notified Bodies.
Look to Rook for additional information on how these fees may apply to your specific device and country to enter the market under MDR or help transition your device from MDD to MDR. To get a head start on the Technical File transition from MDD to MDR, RookQS has partnered with Greenlight Guru to provide a FREE General Safety and Performance Requirements (GSPR) Gap Assessment Tool. This tool will guide in transitioning from the Essential Requirements of the MDD to the new GSPR of the MDR through an easy-to-use method and template.