Blog
%20Developing%20Medical%20Device%20Software%20Cover%20Image.png)
%20Q-Sub%20Process%20Cover%20Image.png)
A Strategic Guide to the Q-Sub Pre-Submission Process
Feb 11, 2026
3 min read

FDA's Updated Medical Device Inspection Program
Feb 03, 2026
4 min read

ISO 9001:2026 Is on the Horizon
Jan 22, 2026
1 min read

5 Medical Device Quality Predictions for 2026
Jan 08, 2026
1 min read
How FDA’s Digital Health Center of Excellence Is Shaping 2026 MedTech Innovation
Nov 26, 2025
3 min read

Leveraging MDSAP to Prepare for QMSR Integration
Nov 26, 2025
8 min read

FDA Expectations for AI/ML Model Training in SaMD (2025 guide)
Nov 25, 2025
3 min read

FDA Issues Draft Guidance on QMS Information for Premarket Submissions
Nov 04, 2025
4 min read

What’s Ahead for Medical Device Regulation: CDRH 2026 Guidance Priorities
Oct 29, 2025
2 min read
-1.png)
Integrating FDA QMSR Requirements into Your Internal Audit
Oct 20, 2025
2 min read
EU AI Act FAQ for Medical Device Manufacturers
Oct 15, 2025
2 min read
.jpeg)
US Government Shutdown: FDA & Medical Device Impact
Oct 09, 2025
2 min read

FDA Computer Software Assurance 2025 Guidance
Sep 25, 2025
4 min read
.png)
Rook Quality Systems Appoints Tyler to Lead Regulatory Strategy
Sep 23, 2025
3 min read

FDA’s 2025 Cybersecurity Guidance for Medical Devices
Sep 23, 2025
2 min read


Medical Device User Fees
Aug 06, 2025
3 min read
FDA Announces Full Recognition of IEC 612326-2-6
Aug 04, 2025
2 min read

ISO 10993‑1:2025 Approved & Pending Publication
Aug 04, 2025
3 min read
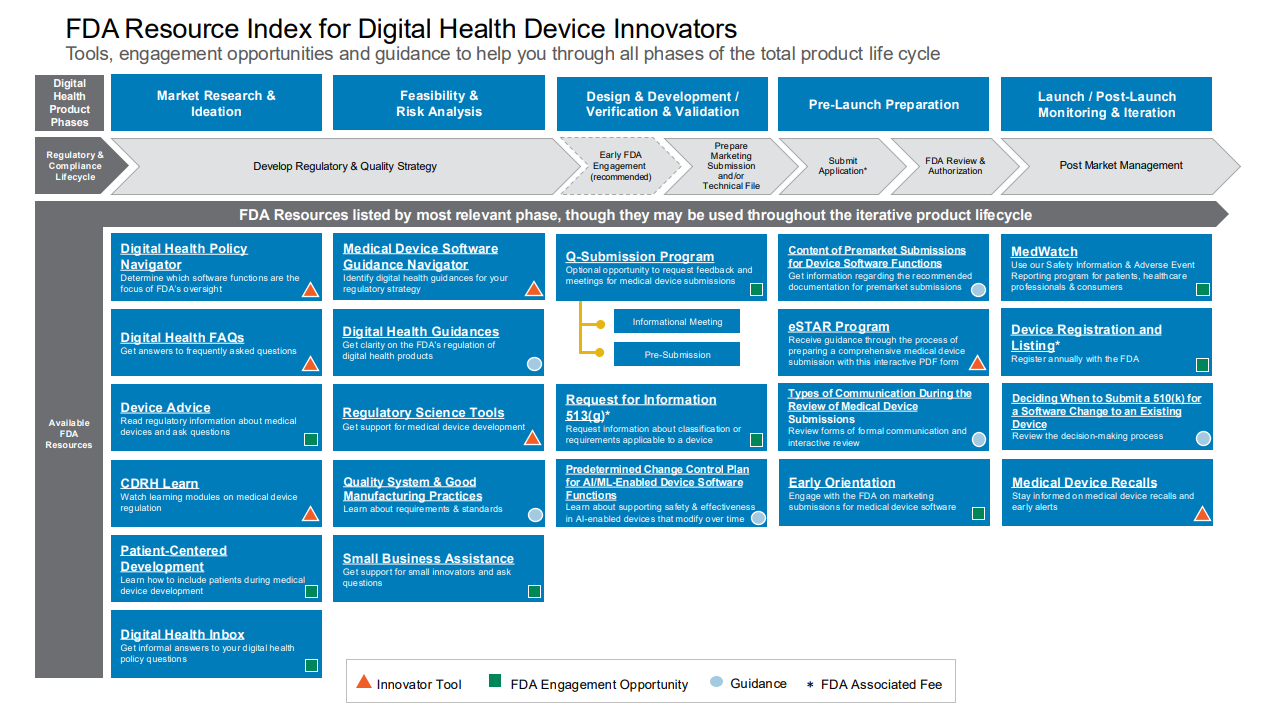
Regulatory Accelerator for Digital Health Innovators
Jul 28, 2025
1 min read
FDA's Retirement of QSIT
May 22, 2025
1 min read

Ensuring the Safety & Effectiveness of AI Systems in Healthcare
May 15, 2025
2 min read

Poor Quality
May 13, 2025
4 min read
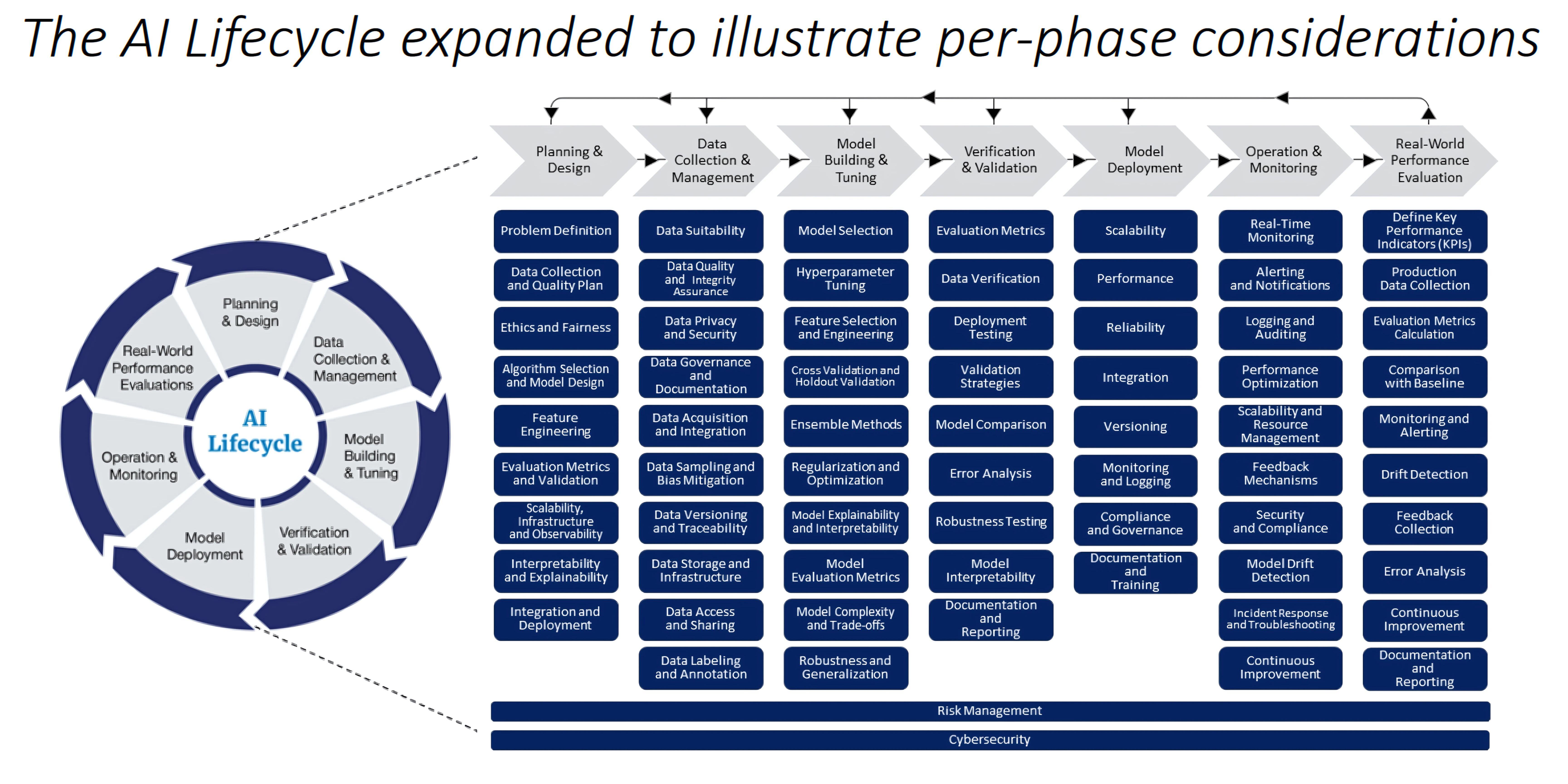
Challenges in Regulating AI-Enabled Medical Devices
Apr 16, 2025
3 min read


Understanding How to Properly Evaluate Suppliers per FDA Requirements 21 CFR 820.50
Oct 25, 2024
3 min read

Small Business Determination Requests Now Accepted Through CDRH Portal
Oct 23, 2024
3 min read
FDA Draft Guidance on Chemical Analysis for Biocompatibility Assessment of Medical Devices
Sep 26, 2024
3 min read

Advancing Innovation in Sterilization Processes: FDA’s Recognition of New Standards
Sep 18, 2024
2 min read

Elevate Your Compliance with Rook Quality Systems’ “Quality as a Service” Offering
Aug 28, 2024
2 min read

Empowering the SDLC with Tools to Bridge R&D and QA Teams
Jul 21, 2024
2 min read

Key Strategies for Successfully Executing the Design Transfer Process
Jul 12, 2024
4 min read

Essential Tips for Preparing for Your ISO 13485:2016 Stage 1 Audit
Jul 11, 2024
3 min read
Mastering Cybersecurity in Medical Devices: Key Strategies and Tools
Jul 10, 2024
2 min read

Understanding the FDA’s Small Entity Compliance Guide for the LDT Final Rule
Jun 26, 2024
2 min read

What is A Pre-Submission (Pre-Sub)?
Jun 10, 2024
3 min read

Keeping Up with the Machine: Predetermined Change Control Plans for Medical Devices
Jun 07, 2024
4 min read

Navigating the Intricacies of Medical Device Software Regulations
Jun 04, 2024
1 min read

FDA Labeling Requirements for Point of Care vs. Home Use Devices
May 10, 2024
2 min read

Navigating the FDA’s Final Rule on Lab-Developed Tests – LDTs
May 03, 2024
2 min read

Navigating Healthcare Cybersecurity: Section 524B of the FD&C Act
Apr 11, 2024
2 min read


A Guide to Etiquette and Preparation for Your First FDA Audit
Mar 08, 2024
1 min read

Guidelines for Verifying Laboratory Testing Data Before FDA Submission
Mar 06, 2024
1 min read

QMSR Final Rule Webinar
Feb 16, 2024

FDA Proposes New Regulations For Laboratory Developed Tests
Feb 13, 2024
1 min read



Understanding the FDA Changes to LDT Regulations
Dec 05, 2023
3 min read

FDA’s Move to Lower 510(k) Submissions to Aid Virtual Health Care
Nov 06, 2023
3 min read

Road to Market for Wearable Medical Devices
Nov 03, 2023
5 min read

The Future Regulation of Laboratory-Developed Tests (LDTs)
Oct 03, 2023
2 min read
![Steps the FDA Continues to Take to Strengthen the Premarket Notification [510(k)] Program](https://rookqs.com/hubfs/Imported_Blog_Media/9_23_23-Blog-Image.jpg)
Steps the FDA Continues to Take to Strengthen the Premarket Notification [510(k)] Program
Sep 20, 2023
3 min read

What To Do (And What Not To Do) When You Receive a FDA Audit Notice
Aug 16, 2023
3 min read

Risk Management in the Medical Device Industry – Part 2
Aug 16, 2023
2 min read

Risk Management in the Medical Device Industry
Aug 01, 2023
3 min read

Where does AI meet the Medical Device Industry?
Jul 06, 2023
3 min read

FDA Releases Updates on Software Guidance
Jun 27, 2023
3 min read

The Costs of Entering the EU Market
Jun 01, 2023

The Partnership Continues: Greenlight Guru to offer RookQS SaMD Templates
May 12, 2023
2 min read

QA/RA Implications of the New ‘Predetermined Change Control Plans’ Guidance
Apr 14, 2023
6 min read

Shining A Light on MDR Notified Body Standard Fees
Mar 23, 2023
2 min read


Medical Device Cybersecurity
Feb 03, 2023
8 min read

How to Prepare Human Factors Documentation for the FDA Premarket Submission
Feb 03, 2023
5 min read

Internal Audits & Annual Physicals: Is Ignorance Really Bliss?
Feb 03, 2023
2 min read


APAC (Asia-Pacific) Medical Device Regulatory Landscape
Dec 09, 2022
3 min read

How to Simplify Medical Device Reporting Set-Up
Dec 06, 2022
2 min read



Leveraging AI for Enhanced Healthcare Delivery
Nov 23, 2022
2 min read

UKCA Mark Delay – How to Capitalize on the Extension
Nov 07, 2022
3 min read
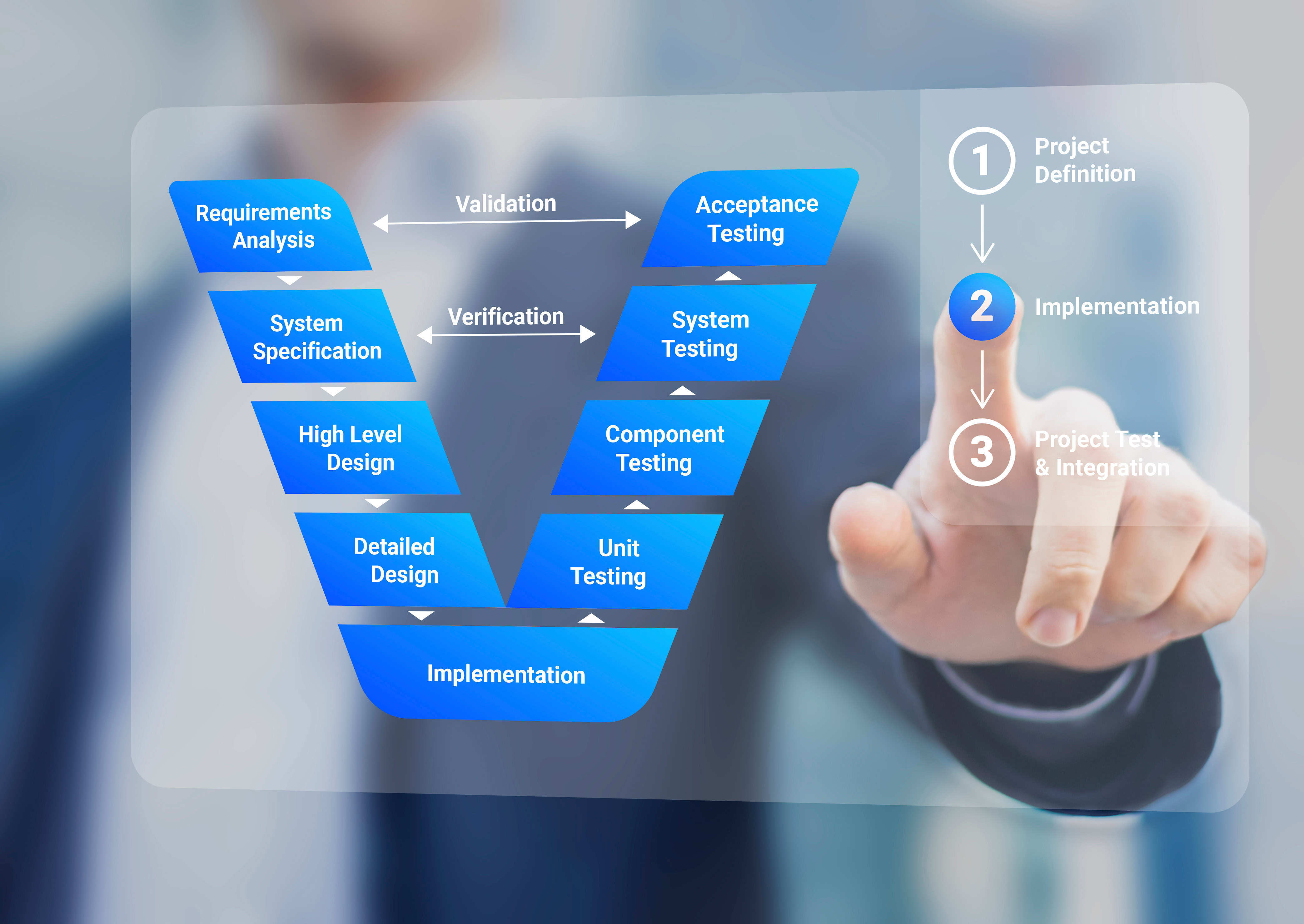
MedTech Software Verification and Validation
Sep 30, 2022
3 min read

Challenges of SaMD (Software as a Medical Device) Product Development
Sep 12, 2022
3 min read


Preparing For Your First FDA or ISO Audit
Aug 09, 2022

FDA Q-Submission Program: What You Need To Know
Aug 05, 2022
3 min read


How To Prepare For Your First FDA Audit
Jun 27, 2022
2 min read

How To Prepare For Your First ISO Audit
May 20, 2022
4 min read

What Should (and Shouldn’t) Go In a Technical File for MDR?
Apr 28, 2022
2 min read
COVID-19 EUA and Enforcement Policy Transition Plans
Apr 12, 2022
4 min read

Rook Quality Systems: Celebrating a Decade of Gratitude and Growth
Mar 20, 2022
3 min read


Peace of Mind At Your Fingertips: Digital Health Funding Explodes
Jan 17, 2022
3 min read



FDA Updates Software Guidance (Part II of II)
Nov 09, 2021
2 min read

FDA Updates Software Guidance (Part I of II)
Nov 04, 2021
2 min read

Align Agile Practices with QMS Requirements
Aug 06, 2021
3 min read

New FDA Guidance Regarding Bench Testing
May 17, 2021
1 min read

Five action items to address Artificial Intelligence/Machine Learning- based SaMD
Apr 20, 2021
2 min read
Software as Medical Device (SaMD) Challenges and Opportunities for 2021 and Beyond Webinar
Jan 11, 2021
2 min read
FDA Digital Health Center of Excellence Listening Sessions
Jan 11, 2021
3 min read

Software Development Process for Healthcare Applications
Nov 09, 2020
2 min read

Enforcement Policy from the FDA to combat COVID-19
Nov 05, 2020
2 min read


Enforcement Policy — Digital Health Devices For Treating Psychiatric Disorders During COVID-19
Nov 05, 2020
3 min read
Key Considerations for SaMD Companies Developing and Commercializing Software as Medical Devices
Nov 05, 2020
1 min read


Regulatory requirements for medical device software
Nov 04, 2020
3 min read

What is Software as a Medical Device (SaMD)?
Sep 09, 2020
1 min read

Classification and Qualification of SaMD under MDR
Jun 01, 2020
3 min read

How do startups leverage FDA’s Pre-Cert Working Model – Part 2
Mar 10, 2020
4 min read

How to Leverage the FDA Pre-Cert Program: Part 1
Mar 09, 2020
3 min read


The Essential Guide to Preparing Your QMS For EU MDR
Sep 20, 2019
1 min read

Importance of Software Testing on Software as Medical Device (SaMD)
May 09, 2019
2 min read


FDA’s take on Digital Health Regulatory Paradigm Shift
Apr 09, 2019
3 min read
Join our mailing list for the latest Rook events, thought leadership, and more.